- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Analysis method for disassembly failure of lithium-ion batteries
2023-09-06
Analysis method for disassembly failure of lithium-ion batteries
The aging failure of lithium-ion batteries is a common problem, and the decrease in battery performance is mainly due to chemical degradation reactions at the material and electrode levels (Figure 1). The degradation of electrodes includes the blockage of membranes and pores on the surface layer of the electrode, as well as the failure of electrode cracks or adhesion; Material degradation includes film formation on particle surfaces, particle cracking, particle detachment, structural transformation on particle surfaces, dissolution and migration of metal elements, etc. For example, the degradation of materials can lead to capacity decay and increased resistance at the battery level. Therefore, a thorough understanding of the degradation mechanism that occurs inside the battery is crucial for analyzing the failure mechanism and extending the battery life. This article summarizes the methods for disassembling aged lithium-ion batteries and the physical and chemical testing techniques used to analyze and disassemble battery materials.
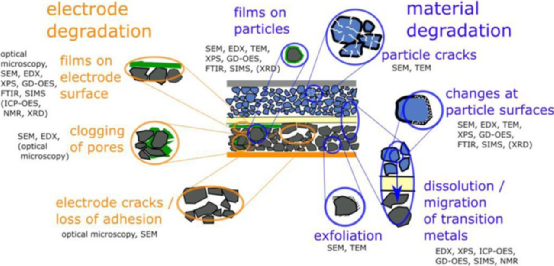
Figure 1 Overview of aging failure mechanisms and common analysis methods for electrode and material degradation in lithium-ion batteries
1. Battery disassembly method
The disassembly and analysis process of aging and failed batteries is shown in Figure 2, which mainly includes:
(1) Battery pre inspection;
(2) Discharge to cut-off voltage or a certain SOC state;
(3) Transfer to a controlled environment, such as a drying room;
(4) Disassemble and open the battery;
(5) Separate various components, such as positive electrode, negative electrode, diaphragm, electrolyte, etc;
(6) Conduct physical and chemical analysis of each part.
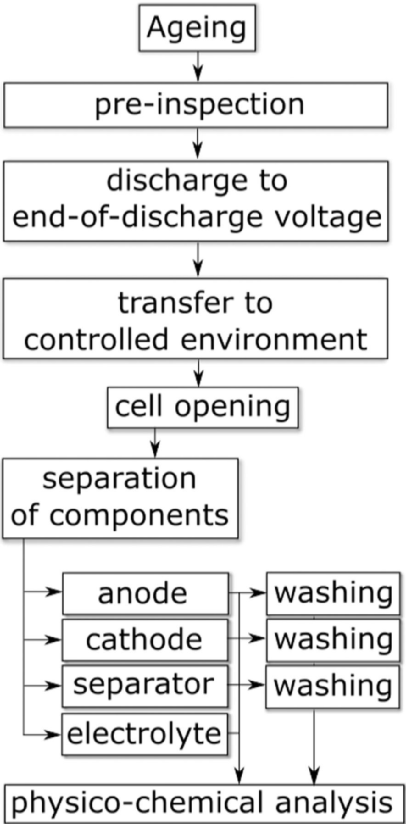
Figure 2 Disassembly and Analysis Process of Aging and Failure Batteries
1.1 Pre inspection and non-destructive testing of lithium-ion batteries before disassembly
Before disassembling cells, non-destructive testing methods can provide a preliminary understanding of the battery attenuation mechanism. Common testing methods mainly include:
(1) Capacity testing: The aging state of a battery is usually characterized by its state of health (SOH), which is the ratio of the battery discharge capacity at time t of aging to the discharge capacity at time t=0. Due to the fact that the discharge capacity mainly depends on temperature, discharge depth (DOD), and discharge current, regular checks of operating conditions are usually required to monitor SOH, such as temperature 25 ° C, DOD 100%, and discharge rate 1C.
(2) Differential Capacity Analysis (ICA): Differential capacity refers to the dQ/dV-V curve, which can convert the voltage plateau and inflection point in the voltage curve into dQ/dV peaks. Monitoring the changes in dQ/dV peaks (peak intensity and peak shift) during aging can obtain information such as active material loss/electrical contact loss, battery chemical changes, discharge, under charge, and lithium evolution.
(3) Electrochemical impedance spectroscopy (EIS): During the aging process, the impedance of the battery usually increases, leading to slower kinetics, which is partly due to capacity decay. The reason for the increase in impedance is caused by the physical and chemical processes inside the battery, such as the increase in resistance layer, which may be mainly due to SEI on the anode surface. However, battery impedance is influenced by many factors and requires modeling and analysis through equivalent circuits.
(4) Visual inspection, photo recording, and weighing are also routine operations for analyzing aging lithium-ion batteries. These inspections can reveal issues such as external deformation or leakage of the battery, which may also affect aging behavior or cause battery failure.
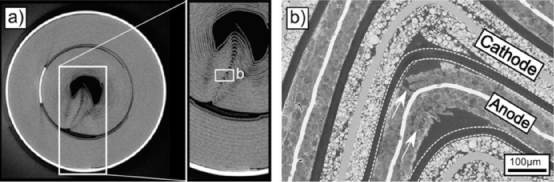
(5) Non destructive testing of the battery interior, including X-ray analysis, X-ray computed tomography, and neutron tomography. CT can reveal many details inside the battery, such as the deformation inside the battery after aging, as shown in Figures 3 and 4.

1.2. Disassembling lithium-ion batteries in a fixed SOC and controlled environment
Before disassembly, the battery must be charged or discharged to the specified state of charge (SOC). From a safety perspective, it is recommended to conduct deep discharge (until the discharge voltage is 0 V). If a short circuit occurs during the disassembly process, deep discharge will reduce the risk of thermal runaway. However, deep discharge may cause unwanted material changes. Therefore, in most cases, the battery is discharged to SOC=0% before disassembly. Sometimes, for research purposes, it is also possible to consider disassembling batteries in a small amount of charged state.
Battery disassembly is generally carried out in a controlled environment to reduce the impact of air and moisture, such as in a drying room or glove box.
1.3. Lithium ion battery disassembly procedure and component separation
During the battery disassembly process, it is necessary to avoid external and internal short circuits. After disassembly, separate the positive, negative, diaphragm, and electrolyte. The specific disassembly process will not be repeated.
1.4. Post processing of disassembled battery samples
After the battery components are separated, the sample is washed with a typical electrolyte solvent (such as DMC) to remove any residual crystalline LiPF6 or non volatile solvents that may be present, which can also reduce the corrosion of the electrolyte. However, the cleaning process may also affect subsequent test results, such as washing that may result in the loss of specific SEI components, and DMC rinsing that removes the insulation material deposited on the graphite surface after aging. Based on the author's experience, it is generally necessary to wash twice with a pure solvent for approximately 1-2 minutes to remove trace Li salts from the sample. In addition, all disassembly analyses are always washed in the same way to obtain comparable results.
ICP-OES analysis can use active materials scraped off the electrode, and this mechanical treatment does not change the chemical composition. XRD can also be used for electrodes or scraped powder materials, but the particle orientation present in the electrodes and the loss of this orientation difference in scraped powder may lead to differences in peak strength.
2. Physical and chemical analysis of materials after battery disassembly
Figure 5 shows the analysis scheme of the main batteries and the corresponding physical and chemical analysis methods. The test samples can come from anodes, cathodes, separators, collectors, or electrolytes. Solid samples can be taken from different parts: electrode surface, body, and cross-section.
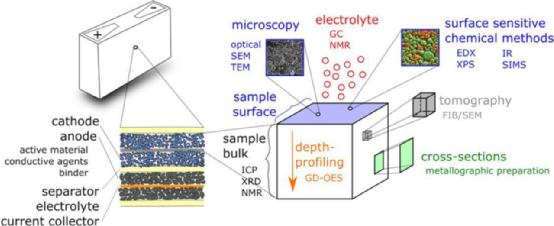
Figure 5 Internal components and physicochemical characterization methods of lithium-ion batteries
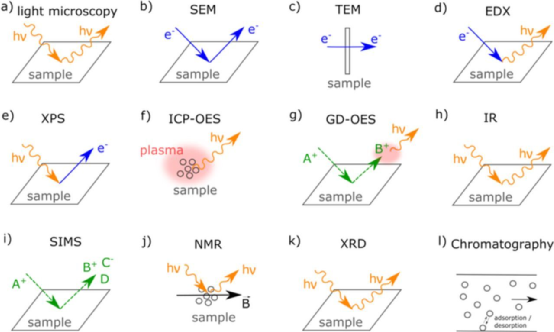
The specific analysis method is shown in Figure 6, including
(1) Optical microscope (Figure 6a).
(2) Scanning electron microscope (SEM, Figure 6b).
(3) Transmission electron microscope (TEM, Figure 6c).
(4) Energy dispersive X-ray spectroscopy (EDX, Figure 6d) is typically used in conjunction with SEM to obtain information about the chemical composition of the sample.
(5) X-ray photoelectron spectroscopy (XPS, Figure 6e) allows analysis and determination of the oxidation states and chemical environments of all elements (except H and He). XPS is surface sensitive and can characterize chemical changes on particle surfaces. XPS can be combined with ion sputtering to obtain depth profiles.
(6) Inductively coupled plasma emission spectroscopy (ICP-OES, Figure 6f) is used to determine the elemental composition of electrodes.
(7) Glow emission spectroscopy (GD-OES, Figure 6g), depth analysis provides elemental analysis of the sample by sputtering and detecting visible light emitted by sputtered particles excited in the plasma. Unlike XPS and SIMS methods, GD-OES deep analysis is not limited to the vicinity of the particle surface, but can be analyzed from the electrode surface to the collector. Therefore, GD-OES forms the overall information from the electrode surface to the electrode volume.
(8) Fourier transform infrared spectroscopy (FTIR, Figure 6h) shows the interaction between the sample and infrared radiation. High resolution data is collected simultaneously within the selected spectral range, and the actual spectrum is created by applying Fourier transform to the signal to analyze the chemical properties of the sample. However, FTIR cannot quantitatively analyze the compound.
(9) Secondary ion mass spectrometry (SIMS, Figure 6i) characterizes the elemental and molecular composition of the material surface, and surface sensitivity techniques help determine the properties of the electrochemical passivation layer or coating on the collector and electrode materials.
(10) Nuclear magnetic resonance (NMR, Figure 6j) can characterize materials and compounds diluted in solid and solvent, providing not only chemical and structural information, but also information on ion transport and mobility, electron and magnetic properties, as well as thermodynamic and kinetic properties.
(11) X-ray diffraction (XRD, Figure 6k) technology is commonly used for structural analysis of active materials in electrodes.
(12) The basic principle of chromatographic analysis, as shown in Figure 6l, is to separate the components in the mixture and then perform detection for electrolyte and gas analysis.
3. Electrochemical Analysis of Recombinant Electrodes
3.1. Reassembling the lithium half battery
The electrode after failure can be electrochemically analyzed by reinstalling the button half battery of lithium. For double-sided coated electrodes, one side of the coating must be removed. The electrodes obtained from fresh batteries and those extracted from aged batteries were reassembled and studied using the same method. Electrochemical testing can obtain the remaining (or remaining) capacity of electrodes and measure reversible capacity.
For negative/lithium batteries, the first electrochemical test should be to remove lithium from the negative electrode. For positive/lithium batteries, the first test should be discharge to embed lithium into the positive electrode for lithiation. The corresponding capacity is the remaining capacity of the electrode. In order to obtain reversible capacity, the negative electrode in the half battery is lithiated again, while the positive electrode is delithized.
3.2. Use reference electrodes to reinstall the entire battery
Construct a complete battery using an anode, cathode, and additional reference electrode (RE) to obtain the potential of the anode and cathode during charging and discharging.
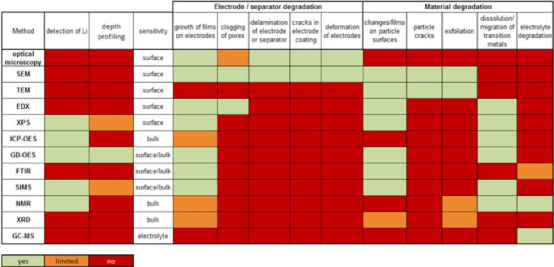
In summary, each physicochemical analysis method can only observe specific aspects of lithium ion degradation. Figure 7 provides an overview of the functions of the physical and chemical analysis methods for materials after disassembly of lithium-ion batteries. In terms of detecting specific aging mechanisms, green in the table indicates that the method has good capabilities, orange indicates that the method has limited capabilities, and red indicates that it has no capabilities. From Figure 7, it is clear that different analysis methods have a wide range of capabilities, but no one method can cover all aging mechanisms. Therefore, it is recommended to use various complementary analysis methods to study samples in order to comprehensively understand the aging mechanism of lithium-ion batteries.
Waldmann, Thomas, Iturrondobeitia, Amaia, Kasper, Michael,et al. Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques[J]. Journal of the Electrochemical Society, 2016, 163(10):A2149-A2164.